Abstract
Introduction: Mesenchymal stromal cells (MSCs) were first explored from the bone marrow in the late 60s and further studies have shown that MSCs are present in several tissues including bone marrow, adipose tissue, umbilical cord, and placenta. Their safety and efficacy were explored in several inflammatory disorders (e.g. acute graft-versus-host disease-aGvHD, Acute respiratory distress syndrome-ARDS) and non-inflammatory diseases (Osteoarthritis and regenerative medicine).
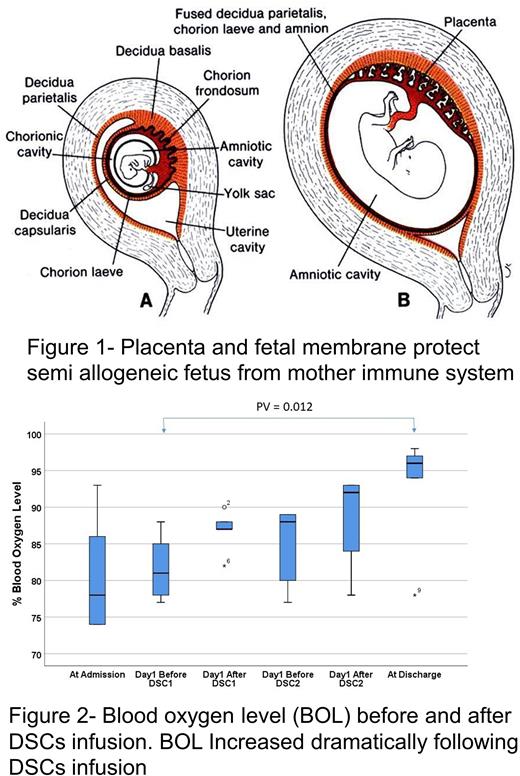
We have expanded this cell therapy approach to newer and more efficient stromal cells, derived from human placenta named decidua stroma cells (DSCs). The fetus is protected against the mother's immune system by the placenta and fetal membranes (Fig1). The DSCs play an important role in the feto-maternal tolerance and have been used in Africa for centuries to treat burns. We have cultured stromal cells from fetal membranes and found that they have a better immunosuppressive effect than stromal cells from other origins. DSCs are safe to infuse with no serious side effects. DSCs differ from BM-MSCs in several aspects; they are half the size, more immunosuppressive, need direct contact to show the immunomodulatory effect, have a better expansion capacity, but do not differentiate well to cartilage and bone. We have so far used DSCs to treat patients with ARDS following Covid-19 infection as well as aGVHD and hemorrhagic cystitis (HC) following HCT. In all treated patients we achieved fairly high positive responses in the vast majority of patients.
Methods The fetal membranes are dissected from the placenta tissues and undergo enzymatic digestion. DSCs are then isolated and expanded under GMP conditions from healthy donors after caesarian section. The isolated DSCs are characterized by flow cytometry, karyotype analysis. Subsequently DSCs are frozen slowly in fetal bovine serum and stored in liquid nitrogen until use. Optimal dose of DSCs is 1 × 106 /kg body weight. To prevent allergic reactions steroids were administered intravenously before each cell infusion. All DSC infusions were administered intravenously.
Results: DSCs were given to ten ARDS patients following Covid-19 infection with pulmonary infiltrate. Eight patients survived and were discharged from ICU, where the blood oxygen level dramatically increased after two DSCs infusions (Behnam et al 2021) (Fig 2). Eleven patients with HC, grade 3-4 were also treated with DSCs after HCT. In 5 patients, HC disappeared within 5 days after DSC infusion. Patients who received DSCs within 3 days after the start of HC had a duration of HC of 5 days and a shorter duration of pain than patients who were given DSCs later (p = 0.02). Nine of the 11 patients (82%) were alive 1 year after HCT (Aronsson-Kurtila et al 2018) . DSCs were also given to 21 patients with severe aGVHD. All patients responded, and one-year survival was 76%. No patient died of GVHD. Cause of death was like those observed in allogeneic HCT patients and no death related to DCS therapy. One-year survival was significantly better than 23% in 22 patients who were treated with BM-MSCs, or 3% for 32 historic controls with conventional therapy (p<0.01) (Ringdén et al 2018). There was no infusion related toxicity. Among more than 200 DSCs infusions we observed only transient reversible infusion reactions in 3 patients. In none of them cell infusion was stopped since no severe side effect was observed ( Sadeghi et al 2019)
Conclusion: MSCs from tissues, like BM, adipose, umbilical cord, and placenta tissues, have taken the step from experimental animals to clinical trials. In this abstract, we have presented the use of DSCs as treatment for acute GvHD, HC and ARDS. Further improvements in clinical efficacy by stromal cells therapy may be achieved by optimizing the cell production process. In future, many different types of MSCs products may be used for specific clinical indications. Primary indications for MSCs and DSCs include among others acute GvHD and hemorrhages, e.g. hemorrhagic cystitis and ARDS, but many other indications, e.g. osteogenesis imperfecta, rheumatic inflammatory disorders, cardiovascular diseases, sepsis and septic shock, inflammatory bowel disease, multiple sclerosis, neuroinflammation and other acute inflammatory disorders may join the line of established therapies.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal